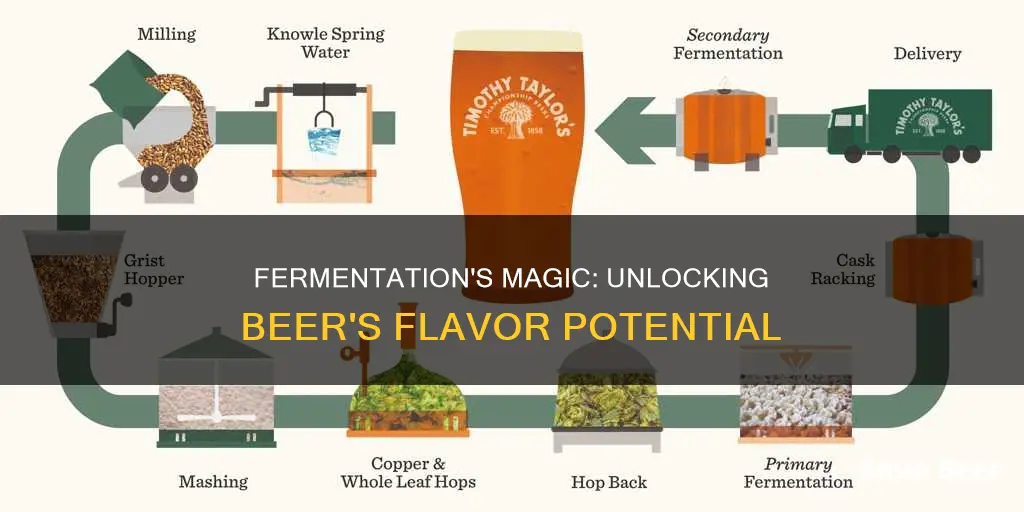
Fermentation is a crucial process in the art of brewing beer, transforming simple ingredients into the beloved beverage we know and love. This natural process involves yeast breaking down sugars derived from malted grains, releasing alcohol and carbon dioxide in the process. The result is a complex interplay of flavors and aromas, ranging from fruity and estery to nutty and roasted, depending on the specific yeast strains and brewing techniques employed. Understanding fermentation is key to unlocking the full potential of beer, allowing brewers to create a wide array of styles and flavors.
What You'll Learn
- Yeast Fermentation: Converts sugars into alcohol and carbon dioxide, creating the beer's alcohol content
- Flavor Development: Fermentation produces esters, phenols, and other compounds that contribute to beer's unique flavors
- Carbonation: Carbon dioxide released during fermentation gives beer its fizz and refreshing quality
- Color Formation: Enzymatic reactions during fermentation can affect the color intensity of the beer
- Preservation: Fermentation inhibits bacterial growth, extending the shelf life of beer

Yeast Fermentation: Converts sugars into alcohol and carbon dioxide, creating the beer's alcohol content
Yeast fermentation is a crucial process in the art of brewing, transforming simple sugars into the complex and delightful beverage we know as beer. This natural process is driven by the yeast, a microscopic organism that plays a pivotal role in the fermentation stage. When yeast encounters the sugars present in the wort (the sugary liquid extracted from malted grains), it initiates a biochemical reaction.
During fermentation, yeast consumes the sugars, primarily maltose, and through a series of metabolic pathways, it breaks down these complex carbohydrates. This breakdown results in the production of two primary byproducts: alcohol and carbon dioxide. The alcohol is what gives beer its characteristic kick, raising the alcohol content, and the carbon dioxide is responsible for the fizz that many beer enthusiasts appreciate.
The yeast's activity can be likened to a miniature factory, where it acts as the catalyst for a series of chemical reactions. These reactions are highly efficient, ensuring that the sugars are fully utilized. As the yeast ferments, it creates a delicate balance of flavors and aromas, contributing to the unique character of different beer styles. For instance, the type of yeast used can influence the beer's flavor profile, with some yeasts producing more esters, which contribute to fruity notes, while others may enhance the beer's dryness or sweetness.
This process is not merely a scientific phenomenon but also an art, as brewers carefully select and manage yeast strains to achieve specific beer styles and flavors. The fermentation process can vary in duration and temperature, allowing brewers to control the beer's final attributes. Longer fermentation periods often result in more complex flavors, while temperature control is essential to ensure the yeast's optimal performance.
In summary, yeast fermentation is the magic that transforms a simple sugar solution into a diverse array of beers. It is a natural process that, when harnessed by skilled brewers, results in the creation of countless beer varieties, each with its own unique story and character.
Exploring the Fun of Beer Funnels: A Unique Experiment
You may want to see also

Flavor Development: Fermentation produces esters, phenols, and other compounds that contribute to beer's unique flavors
Fermentation is a crucial process in the art of brewing, as it transforms simple malt sugars into a myriad of flavors and aromas, resulting in the diverse range of beers we enjoy today. One of the key aspects of this transformation is the production of various compounds that significantly contribute to the beer's unique character. Among these compounds are esters and phenols, which play a pivotal role in flavor development.
Esters are organic compounds formed during fermentation when certain acids react with alcohols. In the context of beer, esters can arise from the interaction of acetic acid and ethanol, creating a fruity and sometimes sweet flavor profile. These esters can contribute to the characteristic fruity notes often associated with styles like Belgian ales and some wheat beers. For instance, the presence of ethyl acetate, a common ester, can impart a distinct apple or banana-like flavor, adding complexity and a refreshing character to the beer.
Phenols, on the other hand, are aromatic compounds that can be derived from the breakdown of certain amino acids during fermentation. These phenols contribute to the beer's overall flavor and can provide a range of sensory experiences. Some phenols may offer a slightly bitter or spicy note, while others can create a more subtle, floral or herbal aroma. The balance of phenols in beer is essential, as too much can result in an overly harsh or astringent taste, while a well-managed level enhances the beer's overall flavor profile.
The production of these compounds during fermentation is a delicate balance of art and science. Brewers carefully select specific yeast strains and adjust fermentation parameters to encourage the desired flavor development. For example, certain yeast strains are known for their ability to produce high levels of esters, making them ideal for creating fruity, aromatic beers. Similarly, specific brewing techniques, such as adding certain hops or malt varieties, can influence the phenolic content, allowing brewers to craft beers with unique and distinct flavors.
In summary, fermentation is a transformative process that significantly impacts the flavor profile of beer. The production of esters and phenols, among other compounds, contributes to the diverse and captivating flavors we associate with different beer styles. By understanding and manipulating these chemical reactions, brewers can create beers with a wide range of flavors, from fruity and sweet to spicy and floral, ensuring there's a beer to suit every palate.
Uncorking the Truth: Can Beer Cause Septic System Issues?
You may want to see also

Carbonation: Carbon dioxide released during fermentation gives beer its fizz and refreshing quality
Fermentation is a crucial process in the art of brewing, transforming simple ingredients like malted grains, water, and hops into the beloved beverage we know as beer. One of the most fascinating outcomes of this process is the creation of carbonation, which plays a pivotal role in the beer's character and appeal.
During the fermentation stage, yeast consumes the sugars present in the wort (the sugary liquid extracted from malted grains) and undergoes a series of biochemical reactions. One of these reactions involves the breakdown of sugars, primarily maltose, into simpler compounds. As a byproduct of this process, yeast releases carbon dioxide (CO2) as a gas. This CO2 is what gives beer its characteristic fizz and contributes to its refreshing nature.
The carbonation level in beer is a result of the balance between the yeast's fermentation activity and the beer's packaging and storage conditions. When the beer is bottled or kegged, the CO2 is dissolved in the liquid, creating a natural carbonation process. This is why opening a bottle of beer often results in a sudden release of bubbles, a testament to the magic of fermentation.
The level of carbonation can vary widely among different beer styles. For instance, lagers typically have lower carbonation, providing a smooth and crisp taste, while wheat beers and some ales often exhibit higher carbonation, adding a lively, refreshing quality. This variation in carbonation is a key factor in the diverse sensory experiences that different beer styles offer.
Understanding the role of fermentation in carbonation is essential for both brewers and beer enthusiasts. Brewers can manipulate fermentation conditions to control the level of carbonation, ensuring their beers meet specific style guidelines. Beer lovers, on the other hand, can appreciate the subtle nuances that carbonation brings to the overall drinking experience, from the initial burst of bubbles to the lingering refreshment that keeps them coming back for more.
The Salty Secret: Why Beer Tasters Add Salt to Draft Beer
You may want to see also

Color Formation: Enzymatic reactions during fermentation can affect the color intensity of the beer
The process of fermentation in brewing is a complex and fascinating journey that significantly influences the final characteristics of the beer, particularly its color. Enzymatic reactions play a pivotal role in this transformation, and understanding these reactions is essential for brewers to achieve the desired color intensity.
During fermentation, yeast metabolizes sugars derived from malted grains, producing alcohol and carbon dioxide. This process is a delicate balance of biochemical reactions. Enzymes, the key catalysts in this equation, are responsible for breaking down complex carbohydrates in the malt into simpler sugars, a process known as saccharification. The efficiency and activity of these enzymes directly impact the beer's color.
One critical enzyme group is the α-amylases, which target starch molecules in the malt. These enzymes convert starches into maltose, a disaccharide that contributes significantly to the beer's color. The more active and abundant these α-amylases, the higher the potential for color development. However, the intensity of the color is not solely dependent on α-amylase activity. Other enzymes, such as β-amylases, also play a role by breaking down maltose into glucose, which can further contribute to color formation.
The duration and temperature of the fermentation process are crucial factors in color development. Longer fermentation times often result in more robust enzymatic activity, leading to increased color intensity. Additionally, specific yeast strains possess unique enzymatic capabilities, influencing the final beer color. For instance, some yeast strains may produce more α-amylases, while others might have enhanced β-amylase activity, resulting in different color profiles.
In summary, the intricate dance of enzymatic reactions during fermentation is a critical aspect of beer production, especially in determining the beer's color. Brewers can manipulate these reactions by adjusting fermentation parameters and selecting specific yeast strains to achieve the desired color intensity, from pale lagers to rich, dark stouts. Understanding these enzymatic processes allows brewers to create a wide range of beer styles, each with its unique visual appeal.
Fermentation Vessel Choice: Jug vs. Bucket for Beer Brewing
You may want to see also

Preservation: Fermentation inhibits bacterial growth, extending the shelf life of beer
Fermentation is a crucial process in beer-making that not only transforms simple ingredients into a delightful beverage but also plays a significant role in preserving the beer's freshness and quality. One of the primary benefits of fermentation is its ability to inhibit bacterial growth, which is essential for extending the shelf life of beer.
During the fermentation process, yeast consumes the sugars present in the wort (the sugary liquid extracted from malted grains) and converts them into alcohol and carbon dioxide. This transformation not only gives beer its characteristic flavor and aroma but also creates an environment that is inhospitable to many bacteria. Yeast, being a living organism, produces byproducts that can act as natural preservatives. For instance, some yeasts produce ethanol, which is a highly effective disinfectant, making it difficult for bacteria to survive in the beer. Additionally, the alcohol content in beer, typically ranging from 2% to 8% ABV, creates an environment that is generally unfavorable for bacterial growth. Bacteria struggle to thrive in such an alcoholic environment, which is why beer can remain stable and palatable over an extended period.
The fermentation process also contributes to the development of beer's unique characteristics, such as its flavor, color, and carbonation. As the yeast ferments the sugars, it produces various compounds, including esters, which contribute to the fruity or spicy notes in certain beer styles. The fermentation temperature and duration can also influence the beer's flavor profile, with warmer temperatures often leading to more rapid fermentation and potentially a more robust flavor.
Moreover, the use of specific yeast strains and fermentation techniques allows brewers to control the outcome of the fermentation process. Some yeast strains are more effective at inhibiting bacterial growth than others. For example, certain ale yeasts, like those used in Belgian or English-style ales, can produce a range of flavors and aromas while also contributing to the beer's preservative qualities. Additionally, the fermentation vessel and temperature control are critical factors. Fermenting at the optimal temperature range for the chosen yeast strain ensures a healthy fermentation process and can further enhance the beer's stability.
In summary, fermentation is a vital process in beer preservation, as it not only transforms the wort into a beverage but also creates an environment that actively inhibits bacterial growth. The alcohol content, yeast strains, and fermentation techniques all contribute to the beer's shelf life, allowing it to remain fresh and flavorful over an extended period. Understanding these fermentation principles is essential for brewers to produce high-quality beer and for consumers to appreciate the art and science behind this ancient craft.
Uncovering the Mystery: Why Beer Often Lands on the Bottom Shelf
You may want to see also
Frequently asked questions
Fermentation is a crucial process in brewing where yeast converts sugars derived from malted grains (usually barley) into alcohol and carbon dioxide. This process occurs in the fermenter, a specialized vessel in the brewing process.
Fermentation plays a significant role in developing the unique characteristics of different beer styles. The type of yeast and fermentation process used can greatly impact the flavor profile. For example, ale yeasts tend to produce fruity esters, while lager yeasts result in cleaner, crisper beers. The duration of fermentation also influences the beer's final taste and aroma.
During fermentation, the yeast consumes the available sugars and produces ethanol (alcohol). The more efficient the fermentation process, the higher the potential alcohol by volume (ABV) of the beer. Brewers carefully manage fermentation to achieve the desired alcohol level and overall beer style.
Fermentation lag refers to a temporary delay in the fermentation process, typically observed when using specialty grains or adjuncts. These ingredients can contain non-maltose sugars that yeast may struggle to metabolize, leading to a slower fermentation rate. Brewers often use specific techniques, such as mashing or adding enzymes, to mitigate this lag and ensure a smooth fermentation.







