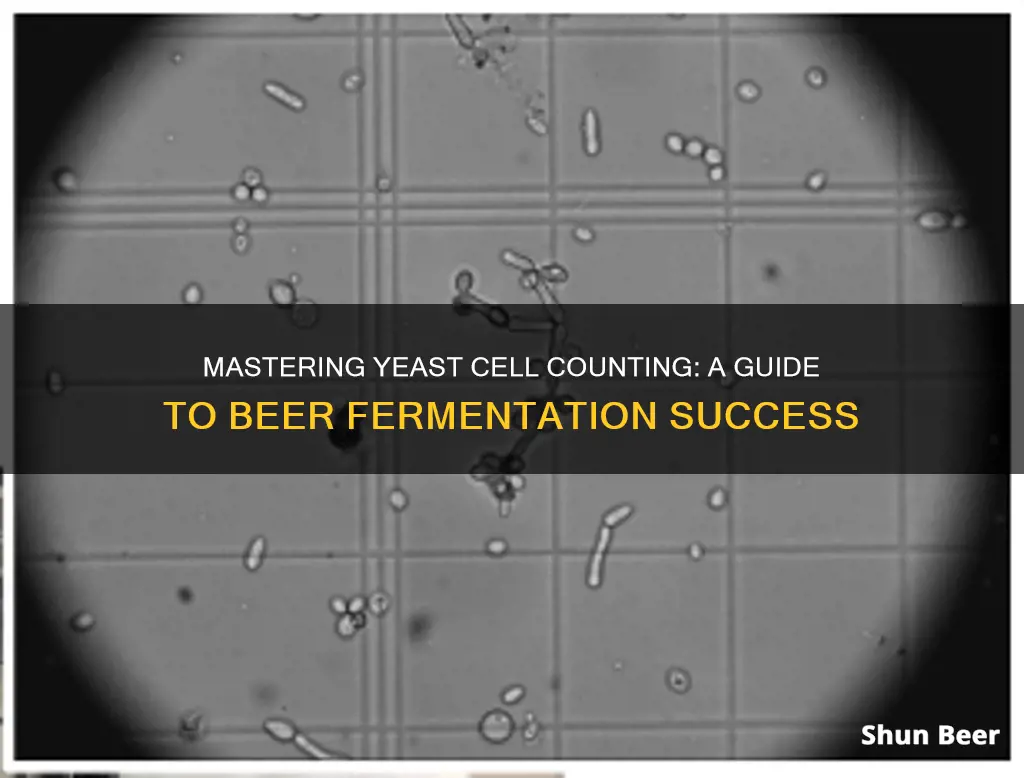
Counting yeast cells in beer is a crucial step in brewing consistent, quality craft beer. Beer scientists need to understand the amount of live and dead yeast cells when pitching specific beers. There are many ways to do this, including using cool machines like the NucleoCounter YC-100, which determines the number of yeast cells and the percentage of living cells (the viability). However, if you want to count cells using a hemocytometer, you will need to dilute a yeast slurry and transfer 1 mL of the diluted yeast slurry into the conical tube, then 1 mL of stain into the same tube.
| Characteristics | Values |
|---|---|
| Dilute a yeast slurry | Yes |
| Use the sample as is | Yes |
| Use a transfer pipette | Yes |
| Allocate 1 millimeter (mL) of diluted yeast slurry into the conical tube | Yes |
| Use a hemocytometer | Yes |
| Count yeast cells | Yes |
| Determine the number of yeast cells | Yes |
| Determine the percentage of living cells (the viability) | Yes |
| Multiply the 1.56E7 cells per mL with 100 | Yes |
| Include the dilution of 1:100 from the beginning | Yes |
What You'll Learn

Counting yeast cells using a hemocytometer
To count yeast cells using a hemocytometer, you will need to dilute a yeast slurry. If you are counting yeast in a fermenter sample or a beer sample, you can use the sample as is. Using a transfer pipette, allocate 1 millilitre (mL) of diluted yeast slurry into a conical tube, then 1 mL of stain into the same tube. Then pipette approximately 10 microliters (µl) of that solution onto the centre of one of the counting areas on the hemocytometer.
Count the number of cells in each of the 10 squares of the hemocytometer. Add all the numbers together and multiply by 5 to get the concentration in 100 nL. Multiply the 1.56E7 cells per mL with 100 to get the final yeast concentration of 1.56E9 cells per mL.
To get the concentration per millilitre (mL), multiply the 1560 cells with 10000. This will give you the concentration per mL. Finally, include the dilution of 1:100 from the beginning.
Unraveling the Magic: Beer's Fermentation Journey
You may want to see also

Diluting yeast slurry before counting
To count yeast cells in beer, you need to dilute a yeast slurry. Here's how:
Use the sample as is if you're counting yeast in a fermenter sample or a beer sample. Using a transfer pipette, allocate 1 millilitre (mL) of diluted yeast slurry into a conical tube, then 1 mL of stain into the same tube. Pipette approximately 10 microliters (µl) of that solution onto the centre of one of the counting areas on the hemocytometer.
If you don't know how to dilute a yeast slurry, watch this video below.
Red Hare Beer: Your Ultimate Guide to Finding This Craft Brew
You may want to see also

Using a NucleoCounter YC-100 to count yeast cells
To count yeast cells using a NucleoCounter YC-100, you will need to dilute a yeast slurry. If you are counting yeast in a fermenter sample or a beer sample, use the sample as is. Using the transfer pipette, allocate 1 millimeter (mL) of diluted yeast slurry into the conical tube, then 1 mL of stain into the same tube. Then pipette approximately 10 microliters (µl) of that solution onto the center of one of the counting areas on the hemocytometer.
The NucleoCounter YC-100 determines the number of yeast cells and the percentage of living cells (the viability).
To start, dilute a yeast slurry. If you don’t know how to do that, watch this video below. If you’re counting yeast in a fermenter sample or a beer sample, use the sample as is. Using the transfer pipette, allocate 1 millimeter (mL) of diluted yeast slurry into the conical tube, then 1 mL of stain into the same tube. Then pipette approximately 10 microliters (µl) of that solution onto the center of one of the counting areas on the hemocytometer.
To get the ideas, google 'how to count yeast cells beer'.
Unibroue Beer: Your Ultimate Guide to Finding This Unique Brew
You may want to see also

Calculating yeast concentration in beer
To calculate the yeast concentration in beer, you will need to count the number of yeast cells and then multiply it by the volume of the sample. Here are the steps:
Firstly, dilute a yeast slurry and use the sample as is if you are counting yeast in a fermenter sample or a beer sample. Using a transfer pipette, allocate 1 millilitre (mL) of diluted yeast slurry into a conical tube, then 1 mL of stain into the same tube. Then pipette approximately 10 microliters (µl) of that solution onto the center of one of the counting areas on the hemocytometer.
Count the number of yeast cells using a hemocytometer. Multiply the number of cells by 5 to get the concentration in 100 nL. Multiply the 1560 cells with 10000 to get the concentration per mL (conversion from 100 nL to mL).
Multiply the 1.56E7 cells per mL with 100 to get the final yeast concentration of 1.56E9 cells per mL. This is the yeast concentration you have in your 1000 mL starter. In total, you now have 1.56E12 yeast cells in the yeast starter (1.56E9 cells per mL x 1000 mL = 1.56E12).
Uncover Hidden Gems: Top Spots for Rare Craft Beer
You may want to see also

Determining yeast viability in beer
Brewing consistent, quality craft beer requires viable yeast. Beer scientists need to understand the amount of live and dead yeast cells when pitching specific beers. There are many ways to do this. There are cool machines like the NucleoCounter YC-100, which determines the number of yeast cells and the percentage of living cells (the viability).
To start, dilute a yeast slurry. If you don’t know how to do that, watch this video below. If you’re counting yeast in a fermenter sample or a beer sample, use the sample as is. Using the transfer pipette, allocate 1 millimeter (mL) of diluted yeast slurry into the conical tube, then 1 mL of stain into the same tube. Then pipette approximately 10 microliters (µl) of that solution onto the center of one of the counting areas on the hemocytometer.
Multiply the 1.56E7 cells per mL with 100 to get the final yeast concentration of 1.56E9 cells per mL. This is the yeast concentration you have in your 1000 mL starter. In total, you now have 1.56E12 yeast cells in the yeast starter (1.56E9 cells per mL x 1000 mL = 1.56E12).
Coming back to the 5 gal (18.9 L) batch of beer with an original gravity of 1.048 (12°). Add all the numbers together: 65+60+70+62+55 = 312 cells. You therefore have 312 cells in 20 nL (since one yellow square equals 4 nL and you counted five in total). Multiply 312 by 5 to get the concentration in 100 nL. In this case, you have 1560 cells in 100 nL. Or 15600 cells in 1 µL. Then multiply the 1560 cells with 10000 to get the concentration per mL (conversion from 100 nL to mL). In this case, 1.56E7 cells in 1 mL.
Uncover the Secrets: Where to Find Yellow Donkey Beer
You may want to see also
Frequently asked questions
To count yeast cells in beer, you need to dilute a yeast slurry. If you don't know how to do that, watch a video on how to do it. If you're counting yeast in a fermenter sample or a beer sample, use the sample as is. Using a transfer pipette, allocate 1 millimeter (mL) of diluted yeast slurry into the conical tube, then 1 mL of stain into the same tube. Then pipette approximately 10 microliters (µl) of that solution onto the center of one of the counting areas on the hemocytometer.
The hemocytometer is a cool machine that determines the number of yeast cells and the percentage of living cells (the viability).
The pitching rate is the initial cell density.
The NucleoCounter YC-100 is a cool machine that determines the number of yeast cells and the percentage of living cells (the viability).
To get the concentration per mL, you need to multiply the 1560 cells with 10000. This will give you 1.56E7 cells in 1 mL.







